European Flavour Regulations Overview
- Regulatory Framework.
- Categories and Definitions of Flavourings.
- Union List of Flavouring Substances.
- Safety Assessment and EFSA’s Role.
- Labelling Requirements.
- Restricted and Prohibited Substances.
- Smoke Flavourings.
- Allergens.
- Functional Additives in Flavourings.
1. Regulatory Framework
The principal legislation governing flavourings in the European Union (EU) is Regulation (EC) No 1334/2008, which came into force on 20 January 2009 and has been applicable since 20 January 2011. This regulation establishes general requirements for the safe use of flavourings and defines different types of flavourings. It also sets out substances requiring evaluation and approval, leading to the creation of the Union list of flavouring substances approved for use in and on foods.
The regulation applies to:
– Flavourings used or intended for use in or on foods
– Food ingredients with flavouring properties
– Foods containing flavourings and/or food ingredients with flavouring properties
– Source materials for flavourings and/or food ingredients with flavouring properties
It does not apply to raw foodstuffs that have not undergone processing, non-compound foodstuffs such as spices and herbs, teas, and infusions, as long as they are consumed as such and not added to food.
2. Categories and Definitions of Flavourings
Regulation (EC) No 1334/2008 defines several categories of flavourings:
– Flavouring substances: Defined chemical substances with flavouring properties.
– Natural flavouring substances: Flavouring substances obtained by appropriate physical, enzymatic, or microbiological processes from material of vegetable, animal, or microbiological origin.
– Flavouring preparations: Products, other than flavouring substances, obtained by appropriate physical, enzymatic, or microbiological processes from food or non-food materials.
– Thermal process flavourings: Products obtained after heat treatment from a mixture of ingredients, at least one containing nitrogen and another a reducing sugar.
– Smoke flavourings: Products obtained by fractionation and purification of a condensed smoke. Note: the EU decided not to renew authorization of all previously approved smoke flavourings, due to genotoxic risks, so they are being phased out.
– Flavour precursors: Products added to food for the sole purpose of producing flavour by breaking down or reacting with other components during food processing.
– Other flavourings: Flavourings that do not fall into the above categories and require evaluation and approval.
3. Union List of Flavouring Substances
The Union list, established by Regulation (EU) No 872/2012, contains flavouring substances approved for use in and on foods. Only substances on this list may be used in the EU. The list includes approximately 2,500 aroma chemicals and is maintained in Annex I of Regulation (EC) No 1334/2008. It is a restrictive list, meaning only those materials on the Union list can be used in flavourings in the EU.
The Union list is periodically updated to include new substances or remove existing ones based on safety assessments conducted by the European Food Safety Authority (EFSA). These updates are implemented through Commission Regulations.
4. Safety Assessment and EFSA’s Role
EFSA is responsible for the scientific evaluation of flavourings. Applicants seeking approval for new flavourings must submit comprehensive data, including information on identity, manufacturing process, compositional data, specifications, stability, proposed uses and use levels, exposure assessment, and toxicological data. EFSA assesses the safety of the flavouring and concludes whether it presents risks to human health.
For certain categories of flavourings, such as those obtained from food and complying with specified production conditions, a safety evaluation may not be required unless there are doubts about their safety.
5. Labelling Requirements
Regulation (EC) No 1334/2008, along with Regulation (EU) No 1169/2011 on the provision of food information to consumers, sets out labelling requirements for flavourings:
– The term ‘flavouring’ can be used in the list of ingredients.
– The term ‘natural flavouring’ may only be used if the flavouring component contains exclusively natural flavouring substances or preparations.
– If a specific source is mentioned (e.g., ‘natural orange flavouring’), at least 95% of the flavouring component must be obtained from the named source, and the flavour must be easily recognisable as that of the source.
– Allergens present in flavourings must be indicated in the list of ingredients, with clear reference to the allergenic substance.
6. Restricted and Prohibited Substances
Annex III of Regulation (EC) No 1334/2008 lists substances that are either prohibited or restricted due to potential health concerns:
– Part A: Substances that shall not be added as such to food.
– Part B: Substances naturally present in flavourings and food ingredients with flavouring properties, for which maximum levels are set in specific food categories.
Examples of restricted substances include:
– Pulegone: Restricted in mint/peppermint-containing confectionery, chewing gum, and beverages.
– Methyleugenol: Restricted in meat preparations, soups, sauces, and non-alcoholic beverages.
– Safrole: Restricted in meat preparations, soups, sauces, and non-alcoholic beverages.
These restrictions are based on toxicological assessments and aim to limit exposure to potentially harmful substances.
7. Smoke Flavourings
Smoke flavourings are regulated separately under Regulation (EC) No 2065/2003, which establishes a Community procedure for the safety assessment and authorisation of smoke flavourings intended for use in or on foods. The regulation ensures a high level of protection of human health and consumer interests. The Union list of authorised smoke flavouring primary products was established by Regulation (EU) No 1321/2013. Note above comment, authorization has not been renewed. It’s important to note they are not immediately banned. The transitional periods are designed to provide manufacturers with sufficient time to reformulate products and find alternative flavouring methods. For consumers and producers in the EU, this means that products containing these smoke flavourings will gradually be phased out over the next few years, with complete removal from the market by 2029 for certain categories.
8. Allergens
Summary is within the table and additional explanation below:
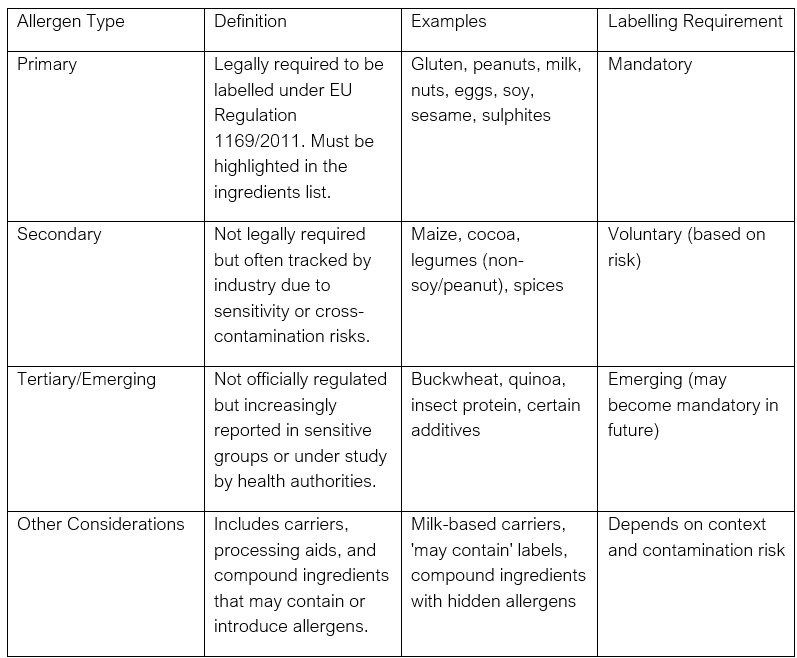
- a) Primary Allergens (EU Regulated)
These are allergens that are legally required to be declared on food labels under EU Regulation (EU) No 1169/2011 (Food Information to Consumers). They must be clearly identified and often highlighted (e.g., in bold) in the ingredients list.
List of 14 Primary Allergens in the EU:
- Cereals containing gluten (wheat, rye, barley, oats, spelt, kamut)
- Crustaceans and products thereof
- Eggs and products thereof
- Fish and products thereof
- Peanuts and products thereof
- Soybeans and products thereof
- Milk and products thereof (including lactose)
- Nuts: almonds, hazelnuts, walnuts, cashews, pecans, Brazil nuts, pistachios, macadamia nuts
- Celery and products thereof
- Mustard and products thereof
- Sesame seeds and products thereof
- Sulphur dioxide and sulphites at concentrations >10 mg/kg or 10 mg/l
- Lupin and products thereof
- Molluscs and products thereof
- b) Secondary Allergens (Industry Monitored)
These are not mandated by legislation to be labelled, but are often monitored due to:
- Cross-contamination risks
- Customer requests
- Sensitive population subgroups
Examples include:
- Maize (corn) – may cause reactions in sensitive individuals, although not a primary allergen
- Cocoa
- Legumes other than peanuts or soy (e.g., lentils, chickpeas)
- Tomato or citrus fruits in flavourings
- Spices (e.g., coriander, garlic, cinnamon) – can cause allergic or intolerance reactions
- c) Tertiary / Emerging Allergens
These include:
Ingredients not yet widely regulated or formally recognised as allergens, but with increasing reports of sensitivity or intolerance.
Often part of emerging allergen surveillance by healthcare bodies or food businesses.
Examples:
- Buckwheat (important in some countries like Japan)
- Quinoa
- Insects/protein isolates from new food technologies
- Specific preservatives or additives (though technically not allergens, they may provoke similar responses)
Other Considerations: Processing Aids and Carriers: While not always allergens themselves, they may introduce allergenic residues (e.g., milk-based carriers in flavourings).
Compound Ingredients: Need careful tracking if they contain sub-ingredients with allergen status.
“May Contain” Statements: Voluntary precautionary labelling to indicate cross-contact risk.
9. Functional Additives in Flavourings
Flavourings may also use functional additives, which are subject to Regulation (EU) No 1130/2011.
Functional additives in flavourings serve roles beyond imparting taste or aroma. They are used to enhance stability, solubility, texture, and shelf life, and to facilitate processing or meet specific dietary needs.
Common Types of Functional Additives
- Emulsifiers
- Function: Help blend oil and water-based components in flavours.
- Examples: Lecithin, mono- and diglycerides, polysorbates.
- Use Case: Common in beverage and dairy flavour formulations.
- Stabilizers
- Function: Maintain flavour integrity over time by preventing separation or degradation.
- Examples: Gum arabic, modified starches, xanthan gum.
- Use Case: Frequently used in spray-dried or encapsulated flavours.
- Carriers and Solvents
- Function: Dilute and disperse flavour components for uniform distribution
- Examples: Propylene glycol, ethanol, triacetin, maltodextrin.
- Use Case: Essential for flavour extracts, powders, and emulsions.
- Antioxidants
- Function: Protect volatile flavour compounds from oxidation.
- Examples: BHA, BHT, tocopherols (Vitamin E).
- Use Case: Used in fat-rich or essential oil-based flavourings.
- Preservatives
- Function: Extend the shelf life of the flavour system.
- Examples: Sorbic acid, benzoates, potassium sorbate.
- Use Case: Especially important in liquid flavour formulations.
- Encapsulation Agents
- Function: Protect sensitive flavour compounds during processing and storage.
- Examples: Modified starches, cyclodextrins, liposomes.
- Use Case: Used in dry flavours, particularly for baked or extruded products.
Benefits of Functional Additives
- Enhanced flavour stability under heat, light, or acidic conditions.
- Improved miscibility of complex flavour systems.
- Controlled release of flavour (e.g. in chewing gum or heat-activated systems).
- Cost-efficiency through extended shelf life and improved processing performance.
Regulatory and Labelling Considerations
Many functional additives are food-grade and regulated by bodies such as EFSA, FDA, and JECFA. They often require appropriate labelling (e.g., E-numbers in the EU), and allergen declarations may be needed depending on the source (e.g., soy lecithin).
June 2025





















